
New Drug Designations - August 2023
Shots:
-
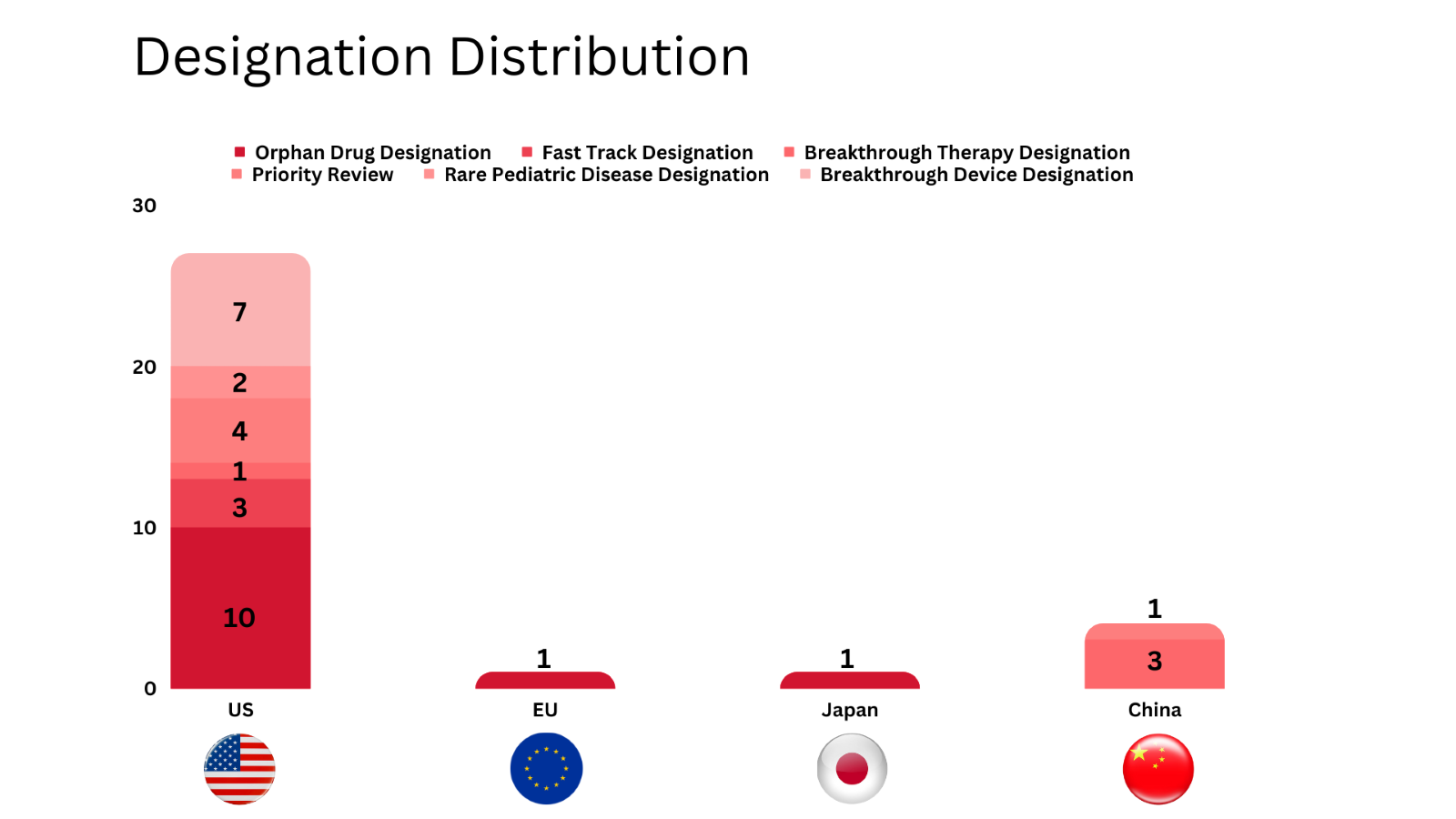
PharmaShots' designation report provides a concise overview of several drugs and their designations by the US FDA, the EU, Japan, and China. This month’s report includes 8 biological drugs, 13 small molecules, 4 cell and gene therapies, 3 diagnostic tests and 5 devices
-
Genprex’ Reqorsa, focused on the treatment of SCLC, is the drug to receive ODD from FDA along with 3 previous FTDs incl. 1 in combination with Tecentriq for ES-SCLC and the other 2 in combination with Tagrisso and Keytruda for NSCLC who progressed after respective treatments
-
PharmaShots has compiled a list of a total of 25 drugs, 3 diagnostic tests and 5 devices awarded with designations by multiple regulatory bodies in Aug 2023

Elraglusib (9-ING-41)

-
Actuate initiated enrollment for a P-II trial evaluating elraglusib (9-ING-41) + gemcitabine/nab-paclitaxel for advanced pancreatic cancer as an expansion arm of the ongoing P-I/II study
-
Furthermore, there are ongoing investigator-led P-II studies for elraglusib in combination with a checkpoint inhibitor (NCT05239182) for front-line treatment of advanced pancreatic cancer patients and in combination with FOLFIRINOX (NCT05077800) for patients with metastatic pancreatic cancer
Zelasudil (RXC007)

-
The topline results from the P-IIa study evaluating zelasudil (RXC007) for the treatment of idiopathic pulmonary fibrosis (IPF) are anticipated in Q1’24
-
Zelasudil (oral) is a highly selective small molecule inhibitor targeting ROCK2 which sits at a nodal point in a cell signaling pathway, believed to be central to fibrosis
LSTA1

-
LSTA1 is being studied in ongoing and planned trials incl. P-Ib/IIa and P-IIb for the treatment of multiple solid tumors in combination with various anti-cancer regimens
-
The company anticipates beginning P-IIa double-blind, PBO-controlled, randomized, PoC trial evaluating LSTA1 with SoC temozolomide vs temozolomide alone in 30 patients with newly diagnosed GBM at Estonia and Latvia. First patient treatment is expected in Q4’23
Reqorsa

-
Reqorsa has previously received 3 FTDs from the US FDA, one in combination with Tecentriq in patients with ES-SCLC and the other two in combination with Tagrisso and Keytruda for the treatment of NSCLC who progressed after respective treatments
-
The P-I/II trial (Acclaim-3) evaluates Reqorsa + Tecentriq in patients with ES-SCLC who did not develop tumor progression after receiving Tecentriq and CT as initial standard treatment
-
The P-I portion will enroll ~12 subjects at the 3-5 US sites to find MTD and the P-II portion will enroll ~50 patients at the 5-10 sites for the treatment with Reqorsa and Tecentriq until disease progression or unacceptable toxicity is experienced
-
The 1EP of the P-II portion is to find 18wks. PFS rate from the time of the start of maintenance therapy with Reqorsa and Tecentriq in patients with ES-SCLC. A P-II futility analysis will be performed after the 25th patient enrolled, and treated reaches 18wks. follow up period
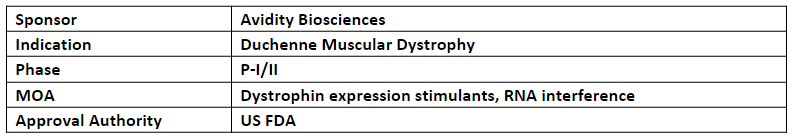
AOC 1044
-
The ODD has been granted to the company's AOC 1044 for the treatment of DMD in people with mutations amenable to exon 44 skipping (DMD44). It also received FTD from the US FDA in Apr’23
-
The P-I/II (EXPLORE44) study evaluates AOC 1044 for the patients with DMD in people with mutations amenable to exon 44 skipping (DMD44)
-
Avidity intends to release findings from the initial phase of the EXPLORE44 trial involving healthy volunteers, during Q4’23. Additionally, the company is actively recruiting individuals who have duchenne muscular dystrophy (DMD44)
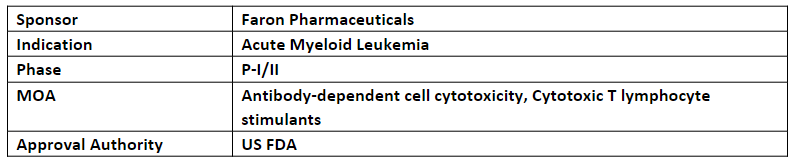
Bexmarilimab
-
The P-I/II (BEXMAB) trial evaluates bexmarilimab + SoC for the treatment of aggressive hematological malignancies of r/r AML and myelodysplastic syndromes (MDS)
-
In Jul’23, the company reported positive results from the P-I portion of the study in which 3 out of 5 patients in the 6 mg/kg bexmarilimab + azacitidine doublet cohort attained objective responses and 8 out of 15 patients achieved objective responses in all three doublet dosing cohorts, with 1 patient remaining on treatment for 13mos.
-
The company expects to complete the dose escalation part, report the results of enrichment cohorts and commencement of the P-II portion of the BEXMAB study in Q4’23
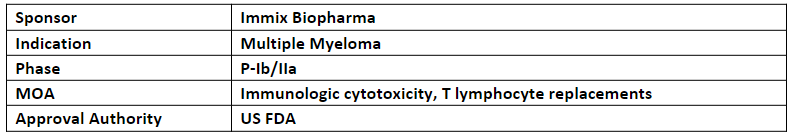
NXC-201
-
NXC-201 is a next generation CAR-T cell therapy that is being evaluated in the P-I (NEXICART-1) study for the treatment of patients with multiple myeloma
NXP800

-
NXP800 is presently undergoing evaluation in a P-Ib study involving patients diagnosed with platinum-resistant, ARID1a mutated ovarian carcinoma. Furthermore, there are plans to extend clinical trials in other medical conditions
-
NXP800 showed significant effectiveness in various preclinical cancer models, incl. ARID1a-mutated ovarian, endometrial and gastric carcinomas, as well as cholangiocarcinoma
ABM-1310

-
ABM-1310 is currently in P-I trials in the US and China for BRAF V600-mutant solid tumors. Interim results from the US P-I study, presented at ASCO’23, showed promising anticancer activity and safety in patients with BRAF V600 mutant tumors, incl. primary brain tumors like GBM. A new P-I trial focusing on GBM has started in China
-
ABM-1310 is an orally administered drug with high selectivity for BRAF mutations, excellent water solubility, and good blood-brain barrier permeability
MAb-AR20.5
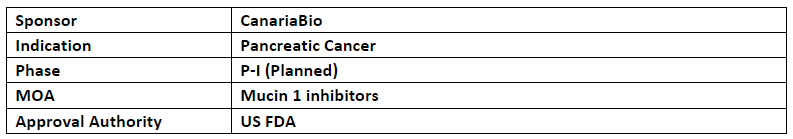
-
CanariaBio anticipates initiating study to evaluate the safety and efficacy of MAb-AR20.5 in patients with pancreatic cancer. The details of which will be available in the coming months
-
MAb-AR20.5 is an IgG1k type murine mAb that binds specifically to the circulating and tumor-associated antigen (MUC1) expressed ubiquitously on pancreatic cancer cells
Mitazalimab

-
In May’23 mitazalimab also received ODD from the US FDA for pancreatic cancer
-
The P-II (OPTIMIZE-1) study evaluates mitazalimab + mFOLFIRINOX for its safety and efficacy in patients with previously untreated metastatic pancreatic ductal adenocarcinoma
-
According to the results from the OPTIMIZE-1 as reported by the company in Jun’23, mitazalimab + mFOLFIRINOX showed improved tumour response and ORR of 57%, an interim ORR of 44% in the full trial cohort (n=57) which is expected to improve with further follow up and m-DOR of 8.7mos. This compares very strongly with an ORR of 31.6% and DOR of 5.9mos. reported in a similar patient population treated with SoC
-
The topline data from the OPTIMIZE-1 trial is anticipated in Q1’24
Elzonris (tagraxofusp)
-
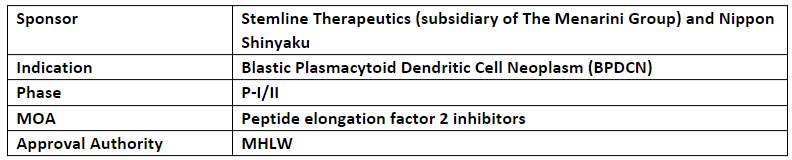
Nippon Shinyaku is conducting P-I/II clinical trial for the development of tagraxofusp in Japan
-
In Mar’21, Stemline entered into an exclusive licensing agreement with Nippon Shinyaku for the development and commercialization of tagraxofusp in Japan with the possibility of co-promotion

Nectero Endovascular Aneurysm Stabilization Treatment (EAST) System

-
The FTD has been granted the Nectero Endovascular Aneurysm Stabilization Treatment (Nectero EAST) System for the treatment of infrarenal AAAs of maximum diameter 3.5 – 5.0 cm
-
The company is conducting the P-II/III (stAAAble) study to evaluate the safety and effectiveness of Nectero EAST System to treat infrarenal AAAs
TSHA-102
-
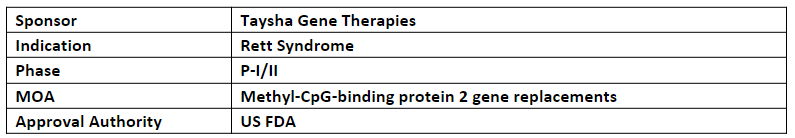
TSHA-102 is the novel miRNA-Responsive Auto-Regulatory Element (miRARE) technology aimed at mediating the levels of MECP2 in the CNS on a cell-by-cell basis without risk of overexpression
-
The company’s TSHA-102 is being assessed in the P-I/II (REVEAL) study in Canada after receiving the IND clearance from the US FDA for pediatric patients with Rett syndrome. The first patient dosing is anticipated in the Q1’24
CBP-4888
-
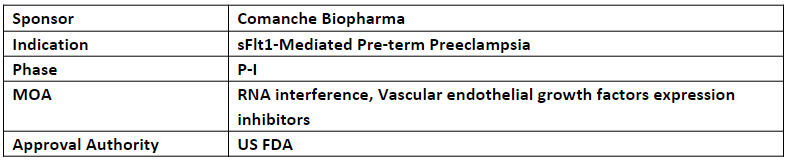
CBP-4888 (SC) is an siRNA therapeutic that is currently being evaluated in the P-I study for the treatment of sFlt1-mediated pre-term preeclampsia

Enhertu

-
The US FDA has granted 2 additional BTDs to Enhertu for adult patients with unresectable or metastatic HER2+ solid tumors and HER2+ (IHC 3+) metastatic colorectal cancer. The therapy is being jointly developed & commercialized by Daiichi Sankyo & AstraZeneca
-
The BTD for metastatic HER2+ solid tumors was based on the P-II study (DESTINY-PanTumor02) in 268 patients with supporting data from other trials in the Enhertu clinical development program demonstrated durable responses, ORR (37.1%), CR (5.6%), PR (31.5%) & SD (46.1%) while DCR in the overall trial population was 68.2%, presented at ASCO’23
-
The BTD for HER2+ metastatic colorectal cancer was based on final results from the P-II trial (DESTINY-CRC01) in 86 patients who showed promising activity & durability with longer-term follow-up. The safety profile was consistent with prior results, presented at ASCO GI'22
Savolitinib

-
The BTD has been granted to savolitinib for the treatment of locally advanced or metastatic G/GEJ adenocarcinoma patients with mesenchymal epithelial transition factor (MET) amplification who have failed at least two lines of standard therapies
-
The single-arm, multi-center, open-label P-II (NCT04923932) trial evaluates savolitinib for its efficacy, safety and tolerability for the treatment of G/GEJ adenocarcinoma in 60 patients with MET amplification
-
The 1EP of the study incl. ORR assessed by IRC and 2EPs incl. PFS and incidence of various AE
Glecirasib

-
The BTD was based on the efficacy and safety results from the trial evaluating gleciracib
-
Glecirasib is currently being evaluated in the pivotal multi-center, single-arm, open-label trial for its safety and efficacy in KRAS G12C-mutated locally advanced or metastatic pancreatic cancer patients who have progressed on frontline SoC
Repotrectinib

-
The BTD has been granted to repotrectinib to treat advanced solid tumors that have an NTRK gene fusion who have progressed following treatment with TRK- TKIs
-
The BTD was based on the results from both global and Chinese NTRK+ TKI-pretreated patients enrolled in the P-I/II (TRIDENT-1) trial
-
Zai lab and Turning Point are together evaluating repotrectinib in two P-I/II trials (TRIDENT-1 and CARE) in pediatric patients. Zai Lab is responsible for the trial in China while Turning Point enrolls patient worldwide
-
Repotrectinib is a next-generation TKI targeting the ROS1 and NTRK oncogenic drivers of advanced solid tumors incl. NSCLC

Xtandi (enzalutamide)

-
US FDA has accepted the sNDA and granted Priority Review of Xtandi in patients with nmCSPC. The US FDA decision is expected in Q4’23
-
The sNDA was based on the results from the P-III trial (EMBARK) evaluating enzalutamide (160mg, qd) + leuprolide, enzalutamide (160mg) as monotx. vs PBO + leuprolide (22.5mg, q12w) in 1068 patients with nmCSPC with high-risk BCR at sites in the US, Canada, EU, South America, and the Asia-Pacific region
-
The study met its 1EPs of MFS the Xtandi + leuprolide & showed a 58% reduction in risk of metastasis or death vs PBO + leuprolide & the overall safety profile was consistent with the known safety profile of each of the medicines. The results were presented at AUA 2023
-
The company will discuss the results with other regulatory authorities globally to support additional license applications in 2023 and beyond. Xtandi’ sNDA is being reviewed under the RTOR program and Project Orbis
Dasiglucagon

-
Dasiglucagon has been awarded priority review status for its use in preventing and treating hypoglycemia in pediatric patients aged 7 days and older who have congenital hyperinsulinism. It applies to a dosing period of up to 3wks. with PDUFA date in Dec’23
-
The NDA review process will be conducted in 2 parts in which part 1 covers dosing up to 3wks., while part 2 focuses on use beyond 3wks
-
Furthermore, the US FDA requested additional analyses from CGM data, expected by year-end, to support its use in CHI beyond 3wks. CGM data was the secondary outcome in one of the pivotal P-III trials
Nefecon
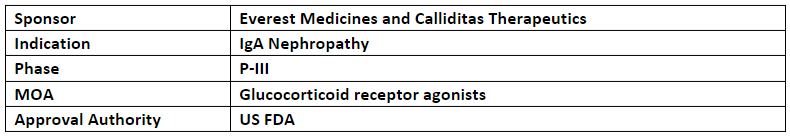
-
The US FDA accepted the sNDA & granted priority review to Nefecon with an expected decision in Dec'23. The sNDA was based on the P-III study (NefIgArd) evaluating Nefecon (16mg, qd) vs PBO in adult patients with primary IgAN on optimized RASi therapy
-
The results showed a significant benefit of Nefecon in eGFR over 2yr. study period consisted of 9mos. of treatment with Nefecon, followed by a 15mos. follow-up period of the study, treatment benefits across the entire study population regardless of UPCR baseline, and difference b/w Tarpeyo & PBO in 2yr. eGFR total slope of ~3mL/min per year. The results were published in The Lancet
-
Nefecon received BTD from the NMPA & was also approved in the US under accelerated approval based on a reduction in proteinuria
Tibsovo (ivosidenib)

-
The US FDA accepted the sNDA and granted priority review to Tibsovo for the treatment of IDH1-mutated r/r MDS
-
The sNDA was based on P-I results, presented at the EHA’23, that showed durable remissions, incl. complete response in nearly 40% of patients, and an acceptable safety profile
-
In the analysis involving 18 patients, Tibsovo demonstrated a CRR: 38.9% and ORR: 83.3%. At the time of data cutoff, the median duration of CR had not been reached and the m-OS: 35.7mos.
-
Among nine transfusion-dependent patients, 66.7% became independent from transfusions during a ≥56-day post-baseline period. TRAEs were consistent with Tibsovo's known safety profile, and no new safety concerns emerged in the study
Ivonescimab

-
The company’s ivonescimab, FIC investigational PD-1/VEGF bi-specific Ab, received priority review of NDA from the China’s NMPA. Ivonescimab has previously received BTD from the NMPA for 3 lung cancer indications incl. NSCLC patients with positive PD-L1 expression, EGFR-mutated, locally advanced or metastatic non-squamous NSCLC and locally advanced or metastatic NSCLC that failed by previous PD-1/L1 inhibitors and Pt-based CT
-
The ivonescimab is currently being evaluated in 4 pivotal registrational P-III studies worldwide as follows:
- A P-III (AK112-303) trial evaluating ivonescimab monotx. vs pembrolizumab monotx. as 1L treatment for NSCLC patients with positive PD-L1 expression
-
An international multicenter P-III (HARMONi/AK112-301) trial evaluating ivonescimab + CT for patients with EGFR-mutated, locally advanced or metastatic non-squamous NSCLC progressing on third-generation EGFR-TKI therapy
-
A P-III (AK112-306) trial in China for the 1L treatment of advanced squamous NSCLC with ivonescimab + CT vs tislelizumab + CT
-
An international multicenter P-III (HARMONi-3) trial evaluating ivonescimab + CT vs pembrolizumab + CT as the 1L for metastatic squamous NSCLC
-
Furthermore, in Dec’22, Akeso and Summit Therapeutics entered into a collaboration and license agreement for ~$5B and Akeso out-licensed exclusive rights to ivonescimab to Summit for the development and commercialization in the US, Canada, EU, and Japan
-
Akeso holds the development and commercialization rights for the rest of the regions incl. China. Ivonescimab is known as AK112 for Akeso at China and Australia, and as SMT112 for Summit's license territories

HG004

-
HuidaGene’s HG004 was granted RPDD by the US FDA for the treatment of inherited retinal disease caused by RPE65 mutations (RPE65-IRDs)
LCTG-001

-
LCTG-001 is a mucosal antibody replacement therapy which comprise of polyclonal IgA for nasal administration in patients with Common Variable Immunodeficiency (CVID)
-
Once LCTG-001 obtains approval, Lactiga becomes eligible to obtain a priority review voucher for future marketing applications

Vanquish System

-
The company is evaluating Vanquish system in a pivotal study (VAPOR 2) in support of an FDA submission for US market clearance, for the treatment of 235 patients with localized, intermediate-risk prostate cancer at 30 sites in the US
-
Vanquish system utilizes thermal energy stored in a few drops of sterile water to deliver targeted treatments to the cancerous tissue through a simple transurethral procedure
-
It is developed to ablate cancer cells while protecting surrounding structures and significantly reducing the likelihood of life-altering side effects common with other prostate cancer treatments
HLA-LOH Companion Diagnostic Test

-
HLA-LOH Companion Diagnostic (CDx) test aims to detect cancer patients with solid tumors who may benefit from treatment with specific targeted therapies when a patient’s tumor has experienced allele-specific loss of heterozygosity (LOH) for specific human leukocyte antigen (HLA) Class I alleles
-
HLA-LOH Companion Diagnostic (CDx) test is based on the machine learning model to analyze sequence data produced by Tempus’ next generation sequencing-based xT CDx assay
AAVrh74 ELISA assay (CDx)

-
The in vitro diagnostic assay detects IgG antibodies to AAVrh74 capsid in human serum, helping identify candidates for Sarepta Therapeutics' gene therapy, ELEVIDYS, used to treat some Duchenne muscular dystrophy patients
-
Quest and Sarepta are expanding their collaboration to develop companion diagnostics for Sarepta's gene therapies, including screening assays for muscular dystrophies like DMD and limb girdle muscular dystrophies (LGMD). The first diagnostic is for ELEVIDYS, Sarepta's FDA-approved gene therapy for DMD, and Quest will also provide clinical lab testing for Sarepta
AngioVac System
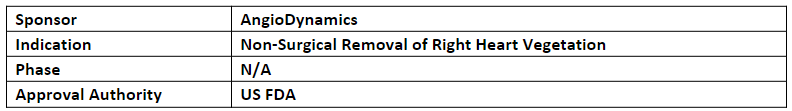
-
The AngioVac System is an on-circuit aspiration system used during extracorporeal bypass for up to 6hrs. It removes thrombi and emboli through a venous drainage cannula, minimizing blood loss via recirculation through the AngioVac bypass circuit. Target vessels include the iliofemoral vein, Inferior Vena Cava (IVC), Superior Vena Cava (SVC), and right heart
GASTROClear

-
The US FDA has granted breakthrough device designation to the company’s PCR-based in vitro diagnostic test, GASTROClear, for early detection of gastric cancer enabling physicians and patients to act early before symptoms occur
-
In 2019, GASTROClear received Singapore HSA’s approval after a trial with >5,200 patients. Mirxes then partnered with 7 Chinese institutions to conduct a trial in 9,000 patients for NMPA registration. The company is planning to expand it in Asia Pacific and considering a US launch
-
GASTROClear is currently accessible in Southeast Asian countries such as Singapore, Indonesia, Malaysia, and the Philippines
J-Valve Transfemoral System
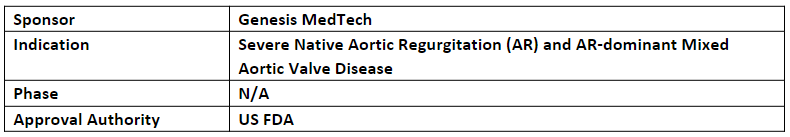
-
The designation has been granted for the treatment of severe native aortic regurgitation (AR) and AR-dominant mixed aortic valve disease in patients who are at high risk for open surgical aortic valve replacement
-
J-Valve TF System is comprised of two key components incl. the J-Valve TF Bioprosthesis and the J-Valve TF Delivery Device. The system utilizes an anchor mechanism and a stent frame that expands to attach the device to a failing valve
NyokAssist Interventional Ventricular Assist Device

-
The NyokAssist utilizes an external motor positioned outside the body, specifically designed to minimize access size and reduce the potential for motor-induced hemolysis due to overheating
Related Post: https://pharmashots.com/15898/new-drug-designations-july-2023
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.













